Harnessing the UK’s genomics expertise to improve patient outcomes
To deliver the potential of genomics for healthcare and research, collaboration and commitment across the sector will be vital.
The ABPI’s first genomics report, 'Harnessing the UK’s genomics expertise to improve patient outcomes', presents the current progress, challenges and opportunities within the UK genomics landscape. It outlines the ABPI’s recommendations to help the UK capitalise on its strengths in genomics, and fully realise the benefits for patient care, our understanding of disease, and the development of future medicines.

Harnessing the UK's genomics expertise to improve patient outcomes
Genomics is an interdisciplinary field of biology focussing on the genetic or epigenetic sequence information of organisms or both in order to understand the structure and function of these sequences and of downstream biological products and processes.
Since the completion of the Human Genome Project in 2003, genomics has been transforming the lives of patients. By providing a more detailed understanding of the genetic causes of disease, it has helped better stratify patient populations, enabling the development of more personalised treatments and supporting earlier diagnosis and prevention. Sequencing the first human genome cost £4billion and took 13 years. Today, it takes less than a day and costs below £1,000.1
Genomics now plays a critical role in understanding whether individuals have an underlying condition (complex or rare), whether they are at risk of developing a condition later in life, and whether they will be responsive or not to a particular treatment.
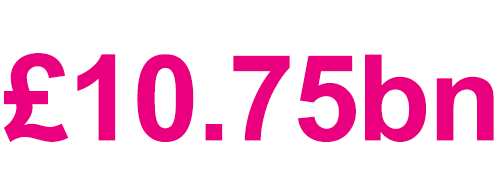
Due to the significant application of genomics in medical research and healthcare, the global market for genomics has grown substantially over recent years and is estimated to grow by £10.75bn between 2021 and 2025.2
The UK has built up global leadership and expertise in genomic capability. This legacy is supported by considerable cross sector expertise, with the Bioindustry Association (BIA), Medicines Discovery Catapult (MDC) and Wellcome Sanger Institute identifying over 140 genomics companies in the UK,3 generating an estimated total turnover of £2.4bn.4 These genomic capabilities underpinned the UK’s response to the pandemic, with the COVID-19 Genomics UK Consortium (COG UK) and a vast diagnostic and sequencing network, putting genomics at the heart of the UK’s research and public health efforts. These genomic capabilities underpinned the UK’s response to the pandemic, with the COVID-19 Genomics UK Consortium (COG UK) and a vast diagnostic and sequencing network, putting genomics at the heart of the UK’s research and public health efforts.
Recognising the UK’s strengths in genomics and the opportunities to unlock further benefits for patients across the country, the UK Government published a national strategy, Genome UK, which outlines how it will maintain and extend the UK’s leadership position. Between 2022 and 2032, the four nations of the UK will work together with the NHS and life sciences sector to realise the potential of genomics for the benefit of patients and ensure that the genomics services thrive in each nation.
From a global pharmaceutical industry perspective, a virtuous cycle is needed, where genomic-enabled R&D drives the delivery of new genomic medicines, which in turn informs the next era of genomic R&D and genomic medicine, to maximise patient benefit. However, too few patients are currently benefiting from the UK’s genomic expertise and assets.
To fulfil the ambition of Genome UK, the UK must drive improvements across the system to translate its R&D expertise and vast infrastructure into improved patient access to genomic medicine.
This report describes both the strengths and weaknesses of the UK’s genomics offer, identifying how the sector can work together to attract further investment and deliver an enhanced offer for patients and their families.
Key facts & figures
The global market for genomics has grown substantially over recent years and is estimated to grow by £10.75bn between 2021-2025. In the UK, genomics related activity has generated an estimated total turnover of £2.4bn over recent years.
As of 28th September 2022, the UK had conducted over 500 million polymerase chain reaction (PCR) tests for SARS-CoV-2 (the virus causing COVID-19) and completed and uploaded over 2 million SARS-CoV-2 genome sequences to the international GISAID database, enabling the identification of current and new variants to inform the public health response to the pandemic.

The 100,000 Genomes Project has to date, led to a rare disease diagnosis for 25% of participants, identified a potential therapy or clinical trial for ~50% of cancer cases and created the National Genomic Research Library (NGRL), which to this day provides data for researchers to learn more about health and disease.

In September 2019, a consortium of government, charity and industry (Amgen, AstraZeneca, GlaxoSmithKline and Johnson & Johnson), came together to fund whole genome sequencing of 450,000 participants, with the aim of deepening the understanding of the interplay between genetic and environmental drivers of health and disease. Two years later, data on the first 200,000 participants was made available to researchers, representing the largest single release of whole genome sequencing data.

The UK’s largest health research programme, Our Future Health has leveraged investment of over £140 million from the life sciences industry, including 8 ABPI member companies, to evolve how disease is detected and potentially at-risk patients identified.

A study conducted by the International Quality Network for Pathology (IQN Path), European Cancer Patient Coalition (ECPC) and European Federation of Pharmaceutical Industries and Associations (EFPIA) found that access to biomarker testing in the UK needed to be improved, in line with best practice in Sweden and Germany, to reduce variability in access to genomic tests and speed-up the availability of genomic tests in conjunction with the introduction of new medicines

The Archangel Newborn Screening Review found that the UK is lagging behind European counterparts, such as Italy, Iceland, and Poland, who screen up to four times as many conditions as the UK, where babies are only tested for 9 conditions. This means that babies in the UK are missing out on critical diagnostic testing and potential life-saving treatments.
Recommendations
Harnessing genomics for research and development
1. The Medical Research Council - UK Research and Innovation (MRC-UKRI) should partner with industry, academia, and charities to scope and deliver the UK Functional Genomics Initiative, ensuring plans build on the UK’s existing expertise and infrastructure.
2. The UK government and NHS should fully deliver the commitments in the Data Saves Lives and Accelerating Genomic Medicine in the NHS strategies, to drive interoperability and connectivity between flagship research programmes and across genomic and health data assets.
3. Genomic medicine services across the four UK nations should work with the research sector to develop a UK-wide Genomic Research Collaborative (see figure 2) which:
i. Supports healthcare systems across the four UK nations in offering the opportunity to participate in genomic research to every individual receiving a genomic test in the NHS.
ii. Monitors and reports on participation in genomic research, promoting diversity and inclusion in recruitment and data collection.
iii. Establishes a framework for genomic research involving clinical and laboratory resources, to ensure ethical and responsible conduct.
4. UKRI should work with industry partners and across research councils to establish an education and training programme for PhD students and post-doctorates to support upskilling in genomic-driven research approaches.
Using genomics to improve disease prevention and patient care
5. Genomic medicine services across the four UK nations should standardise processes, referral pathways and timelines for genomic testing, ensuring testing is delivered in clinically relevant timelines. For England, this should also include coordinated working with Community Diagnostic Centres on processes and flows for diagnostic genomic and pharmacogenomic testing.
6. Genomic medicine services across the four UK nations should publish annual reports on performance metrics and user feedback from healthcare professionals, industry, patients and their families. This should include a comprehensive overview of testing capabilities, turnaround times, and timelines for service improvement, to raise awareness of current and future capabilities.
7. The healthcare systems in all four UK nations should work with industry and regulators to enhance the current horizon scanning function for genomic advances and technologies, to ensure the genomic medicine services adopt new innovations, in line with global trends.
8. Genomic medicine services across the four UK nations should establish a clear process for adding new tests and increasing uptake of newborn screening, ensuring appropriate support is in place for families.
9. Genomic medicine services across the four UK nations should work with Royal Colleges and industry to disseminate and encourage uptake of genomics education materials and resources, including the NHS Genomics Education Programme and Royal College of GPs Genomics Toolkit.
10. The healthcare systems in all four UK nations should incorporate the needs of the genomics workforce in their long-term strategic workplace planning, ensuring there is a recruitment, retention and development strategy to grow the genomic medicine service workforce.
References
- Government Office for Science. Genomics Beyond Health [Internet]. GOV.UK. 2022 [cited 2022 Sep 28].
Available from: https://www.gov.uk/government/publications/genomics-beyond-health - technavio. Genomics Market by Solution and Geography - Forecast and Analysis 2021-2025 [Internet]. Technavio. 2021 [cited 2022 Sep 28].
Available from: https://www.technavio.com/report/genomics-market-industry-analysis - Bioindustry Association (BIA). Genomics nation 2022: Highlighting future opportunities for the UK genomics sector [Internet]. 2022 [cited 2022 Sep 28].
Available from: https://www.bioindustry.org/policy/strategic-technologies/genomics/genomics-nation-2022.html - Office for Life Sciences. Bioscience and health technology sector statistics 2020 [Internet]. GOV.UK. 2022 [cited 2022 Sep 28].
Available from: https://www.gov.uk/government/statistics/bioscience-and-health-technology-sector-statistics-2020/bioscience-and-health-technology-sector-statistics-2020
Last modified: 22 August 2024
Last reviewed: 22 August 2024